Data Insight: Opioid Treatment — Liability Risks in the Office Setting
It is nearly impossible to read or watch the news without mention of the opioid crisis. From patient addiction to death from overdosing, opioid abuse can have tragic outcomes. Further, when these unfortunate events are related to medical care, they can result in malpractice allegations against healthcare providers and organizations.
Across all medical malpractice claims, not just those involving opioids, clinically severe patient outcomes (i.e., outcomes involving permanent injury/disability or death) are noted in 50 percent of cases. In opioid-related claims, however, clinically severe outcomes occur in 70 percent of cases — of these cases, 8 in 10 result in death. Thus, opioid-related claims rank among the malpractice allegations with the highest injury severity. Specific opioids cited in these allegations are varied, but examples include oxycodone, hydromorphone, methadone, fentanyl, and others.
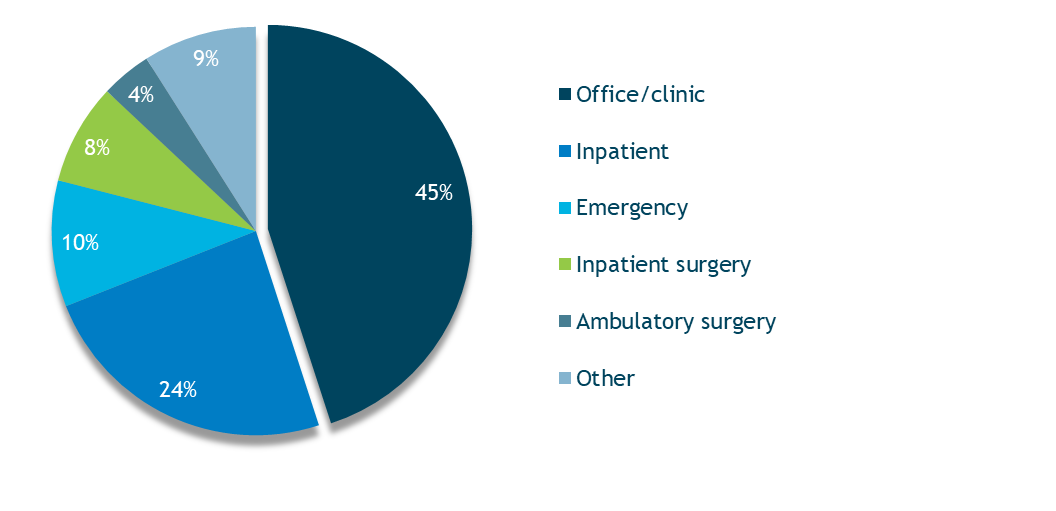
Almost 60 percent of opioid-related malpractice allegations are associated with outpatient settings, with offices/clinics accounting for 45 percent of those claims (Figure 1). Of note, oxycodone, fentanyl, and methadone are the three most frequently cited opioids in these claims.
Figure 1. Opioid-Related Allegations by Location
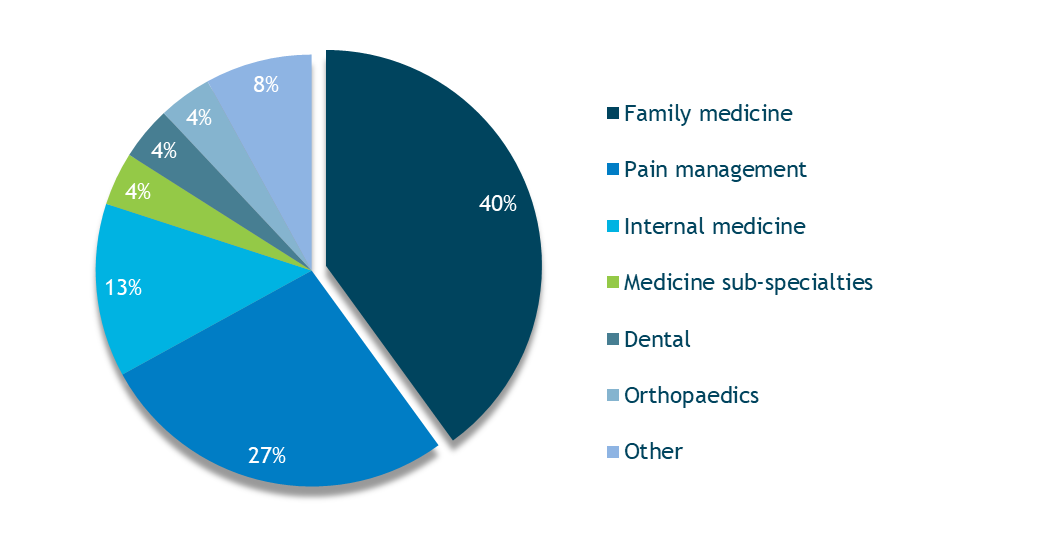
Family medicine providers are identified most often as the responsible clinical service in opioid-related claims associated with offices (Figure 2). Further analysis of these claims reveals that improper management of patients’ pain medication regimens — rather than administration or ordering errors — accounts for the majority of allegations (80 percent) and total dollars paid (90 percent).
Figure 2. Responsible Clinical Services in Opioid-Related Office-Based Claims
Risk Factors
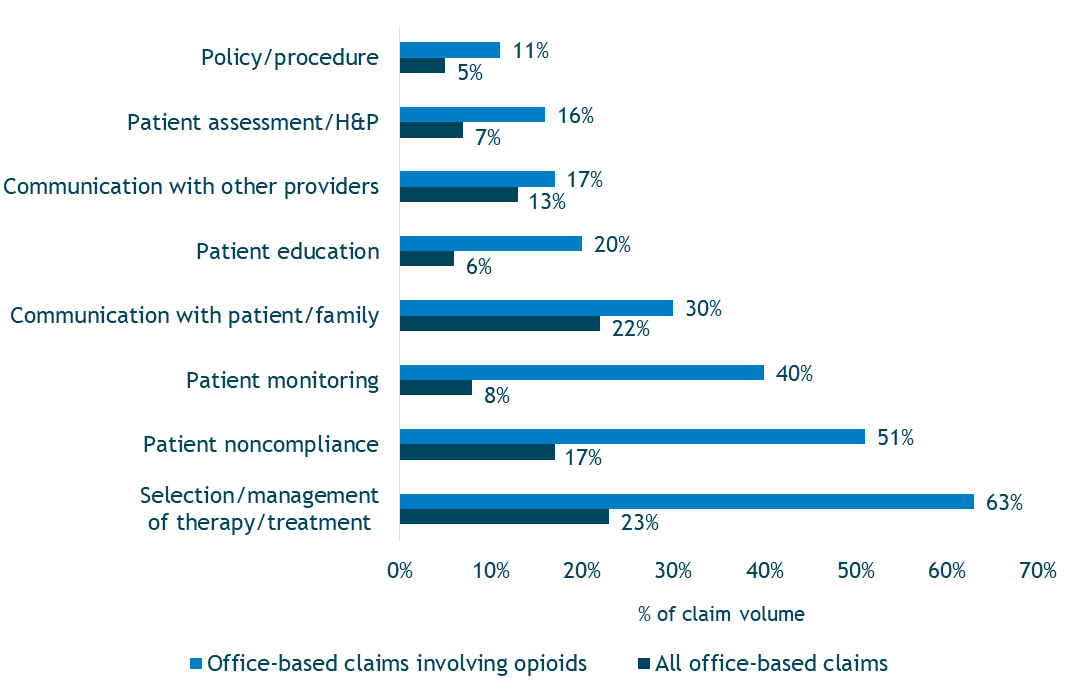
A comparison of opioid-related malpractice claims with other office-based malpractice claims (including diagnosis-related claims and treatment-related claims not linked to medications) reveals several stark differences and helps identify care deficiencies (Figure 3).
A notable deficit in patient care associated with opioid-related claims is a lack of a complete patient assessment prior to prescribing, which would support the selection of opioid therapy. Inadequate documentation of the patient assessment, such as failure to capture details related to patient history and physical (H&P), also is problematic.
Following the prescribing of opioids, inadequate monitoring occurred in 40 percent of cases. Examples of inadequate monitoring include failure to (a) reassess the need for opioid continuation, (b) evaluate patient compliance with treatment plans, and (c) review efficacy of treatment.
Figure 3. Notable Risk Factor Differences in All Office-Based Claims vs. Office–Based Claims Involving Opioids
Patient noncompliance with treatment plans also is cited more frequently in opioid-related claims than in other office-based claims, as is staff noncompliance with established policies and procedures (e.g., ordering refills only after the provider sees the patient). Finally, lack of communication with patients/families about medication risks and inadequate provision of education about treatment also occur more frequently.
Although documentation is not cited as a key differentiator in opioid-related claims, it is a critical risk factor. For patients who are receiving opioid treatment, providers should diligently document all patient assessments (including clinical rationale that supports treatment choice), any communication with the patient, verbal and written patient education, monitoring efforts, and patient compliance with the treatment regimen.
Case Illustration
An obese female patient in her early thirties began seeing a physician for treatment of chronic fibromyalgia pain. Throughout the course of nearly 3 years of treatment, the patient consistently reported that her pain had not subsided. The physician responded by prescribing increasing amounts and types of narcotics, including oxycodone, hydrocodone, and fentanyl. The patient had a past medical history of depression and also was being treated with antidepressants and sleep medications.
The physician ordered a sleep study to evaluate the patient for sleep apnea after her husband reported that she snored heavily. Although no formal diagnosis of sleep apnea was made, the study showed periods of low oxygen saturations. Despite these findings, the patient’s medications were continued.
The patient’s health record contained no documentation of informed consent related to her medications or any patient education about her treatments. The patient’s husband attended one office appointment to report that his wife was "like a zombie." According to the husband, the physician assured him that the patient was receiving appropriate treatment.
With the husband’s support and urging, the patient expressed shortly thereafter that she wanted to stop taking the narcotics. She was started on methadone to wean off the opioids. At that time, the patient’s care was transferred to the office’s nurse practitioner (NP). Although the patient was still taking prescribed narcotics, the physician did not evaluate her again. Further, both the physician and NP often did not document complete information about the patient’s care; they lacked documentation of a physical exam and vital signs for most visits, as well as details about the patient’s history of pain management treatment and her compliance with the prescribed regimen.
Ultimately, the patient’s husband found her dead at home. An autopsy revealed that methadone, oxymorphone, and hydrocodone were present in her bloodstream. The official cause of death was respiratory depression secondary to a combination of narcotic medications. The autopsy also revealed the patient had undiagnosed Hashimoto's disease.
A malpractice suit was filed against the physician and was settled in the high range. The physician also was investigated by the state medical board for excessive prescription of narcotics and failure to appropriately monitor patients.
Key Points
- The majority of opioid-related malpractice claims are associated with outpatient settings, mainly offices/clinics.
- Clinically severe outcomes are noted more frequently in opioid-related malpractice claims than in other types of malpractice claims, and many of these cases involve patient deaths.
- Primary care specialties, particularly family medicine, are identified most often as the primary responsible clinical service in opioid-related malpractice claims.
- Improper management of patients’ treatment regimens, rather than administration or ordering errors, accounts for the majority of opioid-related claims in the office setting.
- Healthcare providers should perform and document thorough patient assessments to support their clinical decision-making and selection of opioid therapy.
- Routine patient monitoring is an essential component of opioid therapy and should be done to assess the need to continue therapy, patient compliance with treatment, and the efficacy of the medication.
Resources
- Checklist: Pain Management
- Opioid Prescribing: Navigating Through a Crisis
- On-Demand Webinar: The Opioid Epidemic: Implications to Managing Care
- Put It in Writing: An Overview of Pain Management Contracts
- Risk Resources: Opioid Prescribing & Pain Management
Data Source
MedPro Group closed claims data, 2007–2016